Stepping into the gummy vitamin market in Canada is an exciting journey, but it comes with its own set of hurdles.
Producing gummy vitamins in Canada requires adherence to Health Canada's Natural Health Products Regulations, obtaining site and product licenses, and selecting equipment that meets Canadian standards. These steps ensure the safe and compliant production of high-quality gummy vitamins.
While this overview provides a foundational understanding, the journey to establishing a gummy vitamin production line involves navigating detailed regulations and making strategic decisions about equipment. Let's delve deeper into each requirement to ensure your venture is set for success.
Health Canada requires a site license for gummy vitamin production.True
Manufacturers must comply with GMP to obtain a site license.
How Do You Obtain a Site License for Gummy Vitamin Production in Canada?
Embarking on gummy vitamin production in Canada involves obtaining a crucial site license. Here's how to secure one.
To obtain a site license for gummy vitamin production in Canada, manufacturers must demonstrate compliance with Good Manufacturing Practices (GMP) as mandated by Health Canada. This involves maintaining proper sanitation, rigorous record-keeping, product testing, and adverse reaction reporting systems.
Understanding Health Canada's Role
Health Canada is the regulatory body overseeing the licensing of sites for gummy vitamin production under the Natural Health Products Regulations (NHPR). Their aim is to ensure consumer safety by enforcing stringent standards on manufacturing practices.
The Importance of Good Manufacturing Practices (GMP)
Adherence to GMP is a critical component in obtaining a site license. It involves:
- Sanitation Protocols: Regular cleaning schedules and protocols to prevent contamination.
- Documentation: Detailed record-keeping for traceability and accountability.
- Quality Control: Implementing systems for consistent quality checks.
Steps to Obtain a Site License
- Prepare Your Facility: Ensure that your production facility meets Health Canada's GMP standards. This includes proper layout, equipment, and personnel training.
- Application Submission: Submit an application to Health Canada that includes detailed documentation of your GMP compliance.
- Inspection Readiness: Be prepared for an inspection from Health Canada officials to verify compliance with all regulatory standards.
Key Documentation Requirements
- Standard Operating Procedures (SOPs): Comprehensive SOPs detailing every aspect of the production process.
- Quality Assurance Reports: Regular reports showcasing adherence to quality standards.
- Adverse Reaction Reporting Systems: Mechanisms to record and report any adverse reactions from consumers.
Common Challenges and Solutions
- Regulatory Complexity: Navigating through complex regulations can be challenging. Consider partnering1 with consultants experienced in Canadian regulatory compliance.
- Documentation Overload: Maintain organized digital systems to handle vast amounts of required documentation efficiently.
- Quality Control: Implement automation where possible to enhance precision and reduce human error in quality checks.
Understanding these elements can significantly ease the process of obtaining a site license. This ensures that your gummy vitamin production not only meets legal requirements but also positions your brand as a trusted provider of health products.
Health Canada oversees site licenses for gummy vitamins.True
Health Canada regulates licensing under the Natural Health Products Regulations.
GMP compliance is optional for site licensing in Canada.False
Adherence to Good Manufacturing Practices is mandatory for licensing.
What Are the Labeling Requirements for Gummy Vitamins in Canada?
Navigating the labeling requirements for gummy vitamins in Canada is crucial to ensuring compliance and consumer safety. Understanding these regulations is key for manufacturers.
Gummy vitamins in Canada must feature a Natural Product Number (NPN) or DIN-HM, detailed ingredient lists, nutritional information, recommended use, and cautionary statements. Compliance ensures consumer safety and adherence to Health Canada's guidelines.
Understanding the Importance of an NPN or DIN-HM
In Canada, every gummy vitamin product must display either a Natural Product Number (NPN) or a Homeopathic Medicine Number (DIN-HM). This number is a testament to Health Canada's approval, indicating that the product has been evaluated for safety, efficacy, and quality. Consumers can trust products with these identifiers as they meet the regulatory standards set by Health Canada.
Manufacturers must apply for these numbers by submitting detailed product information, including ingredient sources and health claims. This process is integral to gaining market access in Canada and assures consumers that the product is safe for consumption.
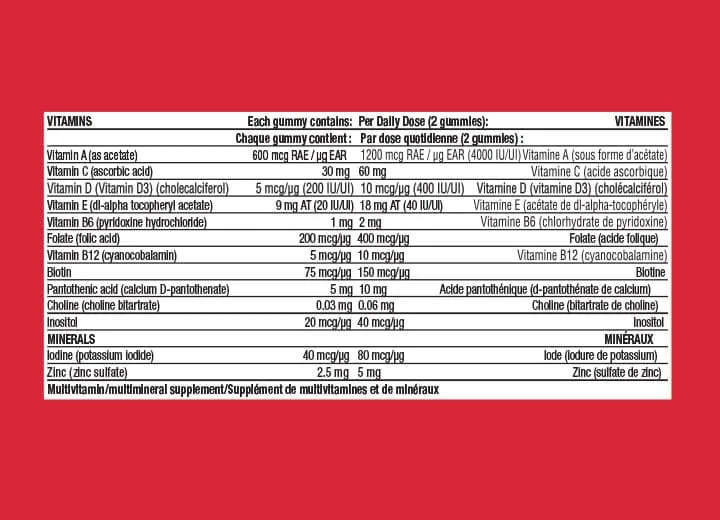
Detailed Ingredient Lists and Nutritional Information
A complete ingredient list is mandatory on gummy vitamin labels in Canada. This transparency allows consumers to make informed choices based on dietary restrictions or allergies. The nutritional information should clearly outline the quantity of active ingredients per serving, including vitamins, minerals, and any additional supplements included in the formulation.
Here's a simplified example of how a label might be structured:
| Ingredient | Amount per Serving |
|---|---|
| Vitamin C | 60mg |
| Vitamin D3 | 25mcg |
| Calcium | 200mg |
| Zinc | 10mg |
Recommendations for Use and Cautionary Statements
Labels must also include recommended usage instructions to guide consumers on safe consumption levels. These instructions help prevent overdosing, especially with fat-soluble vitamins like A, D, E, and K.
Cautionary statements are equally important. They alert users to potential risks or side effects associated with the product. For instance, a gummy vitamin containing high levels of iron should have a warning for people with certain medical conditions.
Compliance with the Food and Drugs Act
Although gummy vitamins fall under the Natural Health Products Regulations (NHPR), they must also adhere to broader regulations outlined in the Food and Drugs Act. This ensures that all health-related products do not mislead consumers through advertising or packaging.
For more information on Health Canada's regulatory framework2, exploring external resources can offer additional insights into the rigorous standards governing health products in Canada.
Ensuring Accurate Labeling
Accurate labeling is not only a regulatory requirement but also a marketing tool that enhances brand trust. By providing clear, concise, and honest information, manufacturers can foster consumer confidence while ensuring compliance with Canadian regulations. Understanding labeling practices3 can significantly impact how consumers perceive and trust your brand.
Meeting these labeling requirements involves careful planning and collaboration with regulatory experts to avoid costly errors and ensure a smooth entry into the Canadian market.
Gummy vitamins in Canada require an NPN or DIN-HM.True
NPN or DIN-HM indicates Health Canada's approval for safety and quality.
Ingredient lists are optional on gummy vitamin labels in Canada.False
Complete ingredient lists are mandatory to ensure consumer safety and compliance.
Why Is UL Certification Important for Gummy Production Equipment?
Navigating gummy production requires understanding the importance of UL certification for equipment. But why is this certification crucial?
UL certification ensures gummy production equipment meets safety standards, enhancing operational credibility and compliance with regulatory inspections. It is a vital practice for maintaining safety and quality in manufacturing environments.

Understanding UL Certification
UL, or Underwriters Laboratories, is a globally recognized safety science company that sets stringent safety standards for products and equipment. In the context of gummy production4, UL certification signifies that the machinery has been thoroughly tested and meets specified safety criteria. This includes electrical safety, mechanical integrity, and fire hazard mitigation.
Enhancing Safety and Compliance
One of the primary reasons manufacturers seek UL certification is to assure compliance with local and international safety standards. This certification provides a layer of assurance that the equipment used in gummy production is less likely to malfunction or pose hazards to operators and the facility. Regulatory bodies often look favorably on operations with UL-certified equipment, as it reflects a commitment to maintaining high safety standards.
Facilitating Regulatory Inspections
For gummy vitamin manufacturers in Canada, regulatory inspections by Health Canada are an integral part of operations. Having UL-certified equipment can significantly ease this process. Inspectors are more likely to approve machinery that has already been vetted by a reputable third party, streamlining the inspection process5 and potentially reducing the time and resources spent on compliance.
Building Credibility with Partners and Consumers
In the competitive world of gummy vitamin production, credibility is key. UL certification not only enhances trust with regulatory bodies but also with business partners and consumers. It signals that a company is committed to quality and safety, which can be a deciding factor for potential partners or customers considering your brand.
Benefits of UL Certification in Manufacturing Environments
- Safety Assurance: Reduces risk of electrical and mechanical failures.
- Operational Efficiency: Minimizes downtime due to equipment-related incidents.
- Market Access: Facilitates entry into markets where UL certification is mandatory or preferred.
| Benefits | Description |
|---|---|
| Safety Assurance | Ensures machinery is less prone to operational hazards. |
| Operational Efficiency | Reduces downtime and improves overall production reliability. |
| Market Access | Enables smoother entry into markets requiring strict safety certifications. |
Strategic Considerations for Manufacturers
While obtaining UL certification may require an upfront investment in terms of time and resources, the long-term benefits make it a worthwhile endeavor. Manufacturers should consider partnering with equipment suppliers who prioritize safety certifications as part of their product offering. This strategic alignment can enhance overall production quality and reliability, positioning your brand as a leader in safe gummy vitamin manufacturing.
UL certification is mandatory for gummy production equipment.False
UL certification is not mandatory but highly recommended for safety.
UL certification enhances credibility with regulatory bodies.True
It shows commitment to safety, easing regulatory inspections.
How Can Automation Enhance Gummy Vitamin Manufacturing Efficiency?
Incorporating automation into gummy vitamin production can revolutionize efficiency, reducing costs and boosting quality.
Automation streamlines gummy vitamin manufacturing by minimizing manual labor, enhancing precision, and ensuring consistent quality. It reduces errors and increases production speed, making operations more cost-effective and reliable.
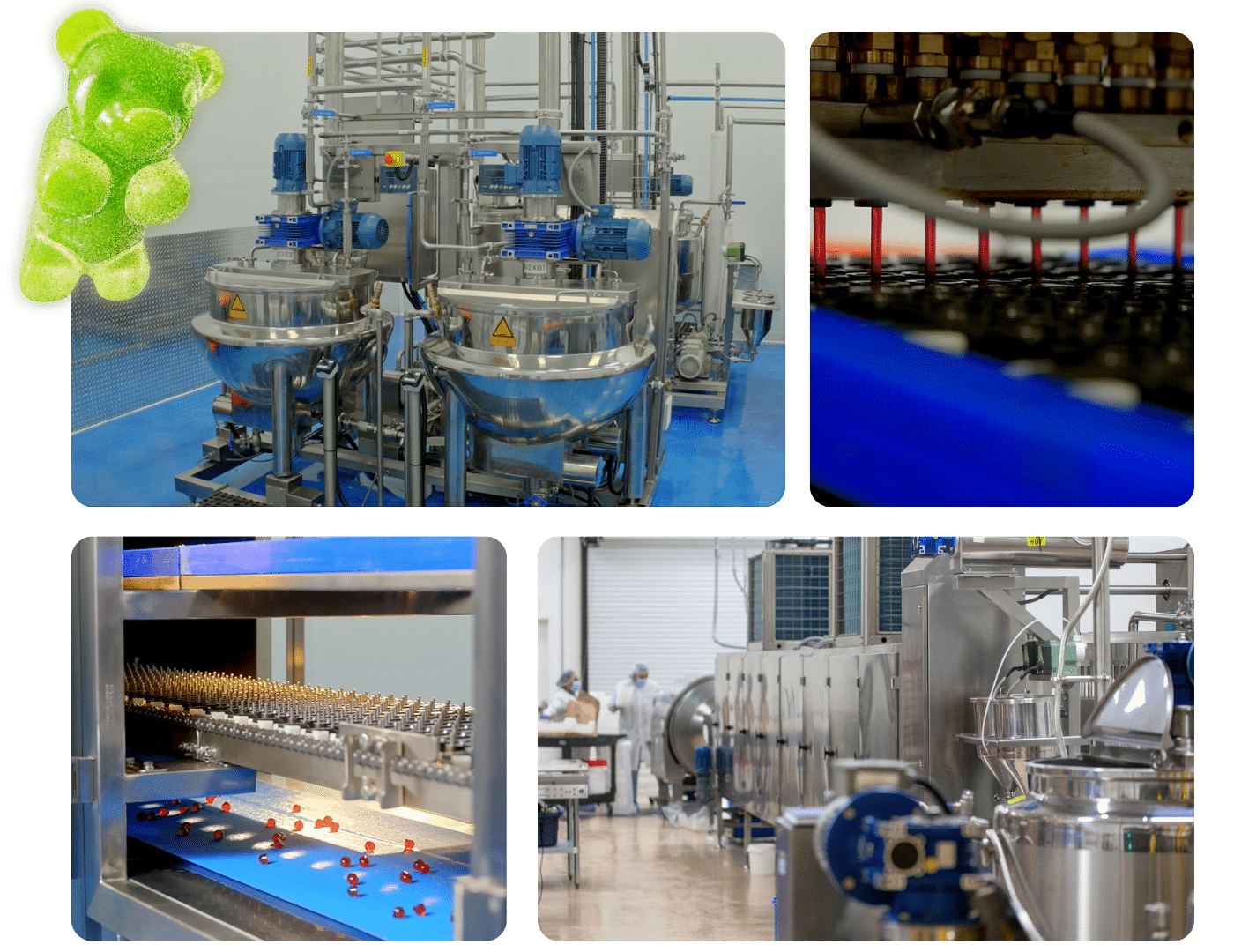
Boosting Production Speed
In the fast-paced world of gummy vitamin manufacturing6, speed is crucial. Automated systems can handle repetitive tasks much faster than manual processes. For instance, automated dispensing systems ensure accurate dosing of vitamins and supplements into each gummy at a pace that manual methods can't match. This increase in speed not only meets high demand but also reduces production time, enabling manufacturers to quickly scale their operations.
Reducing Labor Costs
With labor often being one of the highest expenses in manufacturing, automation presents a solution. By minimizing the need for manual intervention in repetitive or mundane tasks, companies can allocate their workforce to more strategic roles. Automated machines perform tasks such as mixing, molding, and packaging with minimal human oversight, significantly reducing labor costs.
Ensuring Consistency and Quality
Consistency in product quality is non-negotiable in the health supplement industry. Automated machinery excels in maintaining the precision required for consistent product quality. Machines are programmed to execute each step of the production process exactly the same way every time, ensuring each gummy vitamin has the exact dosage as specified by Health Canada's guidelines7. This level of precision reduces the risk of recalls due to inconsistencies and enhances consumer trust.
Enhancing Traceability and Compliance
Automated systems facilitate better traceability throughout the production line. With advanced software, each batch can be tracked from raw materials to the final product. This traceability not only helps in maintaining stringent compliance with regulations but also simplifies the process of identifying and addressing any issues that arise.
Minimizing Human Error
Manual processes are prone to errors that can compromise product quality and safety. Automation reduces these risks by minimizing the reliance on human input for critical tasks. Machines operate with high accuracy, drastically reducing the probability of errors in dosing or mixing, thereby ensuring a safer and more reliable product.
Improving Energy Efficiency
Modern automated equipment is designed to be energy-efficient, which aligns with both cost-saving measures and environmental compliance. For instance, energy-efficient machines reduce operational costs while adhering to environmental regulations regarding energy consumption and emissions. This dual benefit is especially important in Canada, where sustainability is a key concern in manufacturing.
Flexibility and Scalability
Automation provides flexibility in manufacturing processes. Modern equipment can be adjusted to accommodate different formulations or production volumes with minimal downtime. This flexibility is vital for manufacturers aiming to adapt quickly to market demands or introduce new products efficiently.
In summary, integrating automation into gummy vitamin manufacturing not only enhances efficiency but also supports compliance with stringent Canadian regulations, ensuring high-quality products reach the market reliably.
Automation reduces labor costs in gummy vitamin production.True
Automation minimizes manual tasks, lowering labor expenses significantly.
Manual processes ensure more consistent gummy vitamin quality.False
Automation provides precise, consistent dosing, enhancing product quality.
Conclusion
Setting up a compliant gummy vitamin production line in Canada requires not only navigating Health Canada’s regulations but also choosing equipment that meets the highest standards for quality and efficiency. From securing site and product licenses to ensuring accurate labeling and GMP compliance, these steps are essential to producing safe, high-quality gummy vitamins.
With GummyGenix by SaintyCo, you’re backed by over 23 years of pharma grade machine manufacturing experience and a full suite of tailored solutions. Our advanced gummy manufacturing equipment can be customised for the Canadian market, supporting every stage of your production with compliance-focused machinery, expert guidance, and reliable technical support.
Ready to launch or enhance your gummy production? Contact GummyGenix by SaintyCo today to discover how our solutions can elevate your production line for lasting success in the Canadian market. Let’s make your vision a reality—together.
-
Explore expert assistance in navigating Canadian regulatory complexities.: To date, we have completed over 2,000 regulatory filings to Health Canada and the U.S. Food and Drug Administration (FDA) with a 97% success rate. ↩
-
Learn about Health Canada's standards governing health products.: All NHPs are regulated under the Natural Health Products Regulations, which is a separate and distinct regulatory framework from prescription ... ↩
-
Discover effective labeling strategies to build consumer trust.: You must only use one brand name per product per paragraph 5 (e) of the Regulations, as set out in your product's terms of market authorization. ↩
-
Explore how UL certification impacts manufacturing safety standards.: High Standards: A UL mark on a product means the product has undergone rigorous testing to ensure it meets UL's high level of standards for ... ↩
-
Discover how UL certification simplifies inspection processes.: We can review plans, perform on-site evaluations to verify compliance with installation codes, conduct vital checks for critical safety issues, and assess the ... ↩
-
Explore how automation accelerates gummy vitamin production efficiently.: This article discusses the history of gummy candies, the manufacturing process of gummy vitamins, and which machines are ideal to use. ↩
-
Understand guidelines for ensuring product consistency and quality.: All NHPs are regulated under the Natural Health Products Regulations, which is a separate and distinct regulatory framework from prescription drugs. ↩







