Are you looking to launch a gummy manufacturing facility in Australia? Let’s make it a success!
Setting up a compliant supplement or OTC gummy facility in Australia requires TGA registration and GMP standards. You’ll need to secure the right licenses, prioritize product quality and safety, and follow all labeling and advertising guidelines.
Stay tuned as we dive into TGA compliance essentials, choosing smart machinery, and tips from industry experts to streamline your setup. This goes beyond meeting regulations—it's about building a sustainable foundation for your business.
TGA registration is mandatory for all gummies in Australia.True
TGA registration ensures compliance with safety, quality, and efficacy standards.
What Are the Key Steps for TGA Registration?
Looking to see your gummies on Aussie shelves? TGA registration is your golden ticket.
To register products with the TGA, manufacturers must follow a series of steps including product classification, compiling supporting documentation, and ensuring compliance with safety, quality, and efficacy standards. This process is crucial for legal market entry in Australia.

Understanding TGA Registration
Navigating Therapeutic Goods Administration (TGA) registration is much like perfecting a recipe—skip a step, and the outcome won’t be as planned. The process is straightforward but requires careful attention to each detail.
Step 1: Classify Your Product
First, determine whether your product needs to be listed or registered. Listing1 is for lower-risk items, while registration is for those making specific therapeutic claims. This classification is essentially your product’s entry pass to the Australian market.
Step 2: Prepare Comprehensive Documentation
Compile all essential documentation to demonstrate your product’s safety, quality, and efficacy. This includes clinical data, manufacturing information, and more. Think of it as a detailed resume for your gummy products.
Step 3: Adhere to GMP Standards
Your manufacturing site must meet Good Manufacturing Practice (GMP) standards, covering aspects from staff training to equipment upkeep. Consider GMP as the hygiene standard that keeps your production line pristine and compliant.
Step 4: Submit Your Registration
File your application through the TGA portal, along with the appropriate fees. Expect TGA specialists to conduct a thorough review, scrutinizing every detail to ensure compliance.
Step 5: Maintain Compliance and Monitor
Approval isn’t the end—keep monitoring your product for any adverse effects and report these to the TGA. This ensures continued compliance and consumer safety after your gummies reach the market.
Understanding GMP2 is essential in maintaining compliance throughout this process. It's not just about getting approval; it's about staying approved.
Common Mistakes and How to Avoid Them
Overlooking required documentation can cause delays, and underestimating the importance of ongoing monitoring once your product is on the market can lead to compliance issues. Following these steps diligently helps secure a solid foundation for success in Australia’s competitive market.
Following these steps diligently not only ensures compliance but also sets a solid foundation for success in the competitive Australian market.
TGA registration is optional for market entry in Australia.False
TGA registration is mandatory for legal market entry in Australia.
GMP compliance is required for TGA registration.True
GMP compliance ensures product safety, quality, and efficacy standards.
How Does GMP Compliance Impact Your Gummy Manufacturing?
Working through the gummy production maze? GMP compliance is your roadmap to consistent quality and success.
GMP compliance in gummy production ensures consistent quality, safety, and efficiency. It influences every facet of manufacturing, from personnel training to equipment maintenance, ultimately safeguarding consumer trust and regulatory approval.

Enhancing Quality and Safety in Gummy Production
In gummy production, every piece must meet strict safety and quality benchmarks. Good Manufacturing Practice (GMP) is more than just a set of regulations—it’s your guide to achieving these standards. By adhering to GMP, you create gummies under consistent, controlled conditions, reducing risks of contamination or error.
Think of GMP as the conductor for your production symphony. When each step follows its guidance, the result is seamless: high-quality gummies that consumers can rely on. This not only strengthens your brand reputation but also ensures compliance with regulatory bodies like Australia’s Therapeutic Goods Administration (TGA).
Streamlining Processes
GMP compliance brings structure and efficiency to your operations. Standardizing processes means less guesswork and greater precision, reducing waste and boosting overall productivity.
Consider the machinery aspect. With GMP-compliant equipment3, every step from mixing to packaging follows a clear protocol. This setup is akin to having a well-oiled machine, literally and figuratively, where efficiency and reliability are the norms.
Training: The Human Element
Your team forms the core of your production line. Under GMP guidelines, training is a continuous process—not a one-time activity. Employees learn best practices, gaining an understanding of not only how to perform their tasks but also why they matter.
This approach empowers staff, transforming them from mere operators into proactive quality control stewards. It fosters a culture of continuous improvement, enabling team members to identify and address potential issues before they become serious.
Regulatory Harmony
Aligning with GMP isn't just about dodging penalties; it's about achieving harmony with regulatory bodies. Compliance with the Australian Code of GMP for Medicinal Products ensures your operations meet both local and international standards.
By maintaining this alignment, you can confidently expand your gummy empire beyond Australia’s borders. It opens doors to global markets4 where stringent standards are the norm, not the exception.
The Cost-Benefit Equation
While achieving GMP compliance requires an initial investment in time and resources, the returns are substantial. Consistent quality reduces recalls and customer complaints, while efficient processes cut operational costs.
Moreover, being known for high standards can be a selling point, attracting clients who value reliability and excellence in their products. In essence, GMP compliance is your ticket to a sustainable and profitable business journey.
GMP compliance ensures consistent quality in gummy production.True
GMP compliance standardizes processes, ensuring uniform quality and safety.
Training under GMP guidelines is a one-time event.False
GMP requires ongoing training to maintain high standards and adapt to changes.
Which Machines Are Best for Gummy Manufacturing?
Choosing the right machines for your gummy factory is like picking the perfect partner for a dance—precision, rhythm, and adaptability are key.
The best machines for gummy manufacturing are those that align with production scale, ensure precise dosing, and allow adaptability. Startups benefit from flexible gummy machinery for small batches, while larger enterprises need high-capacity, automated gummy production lines with features like real-time monitoring.
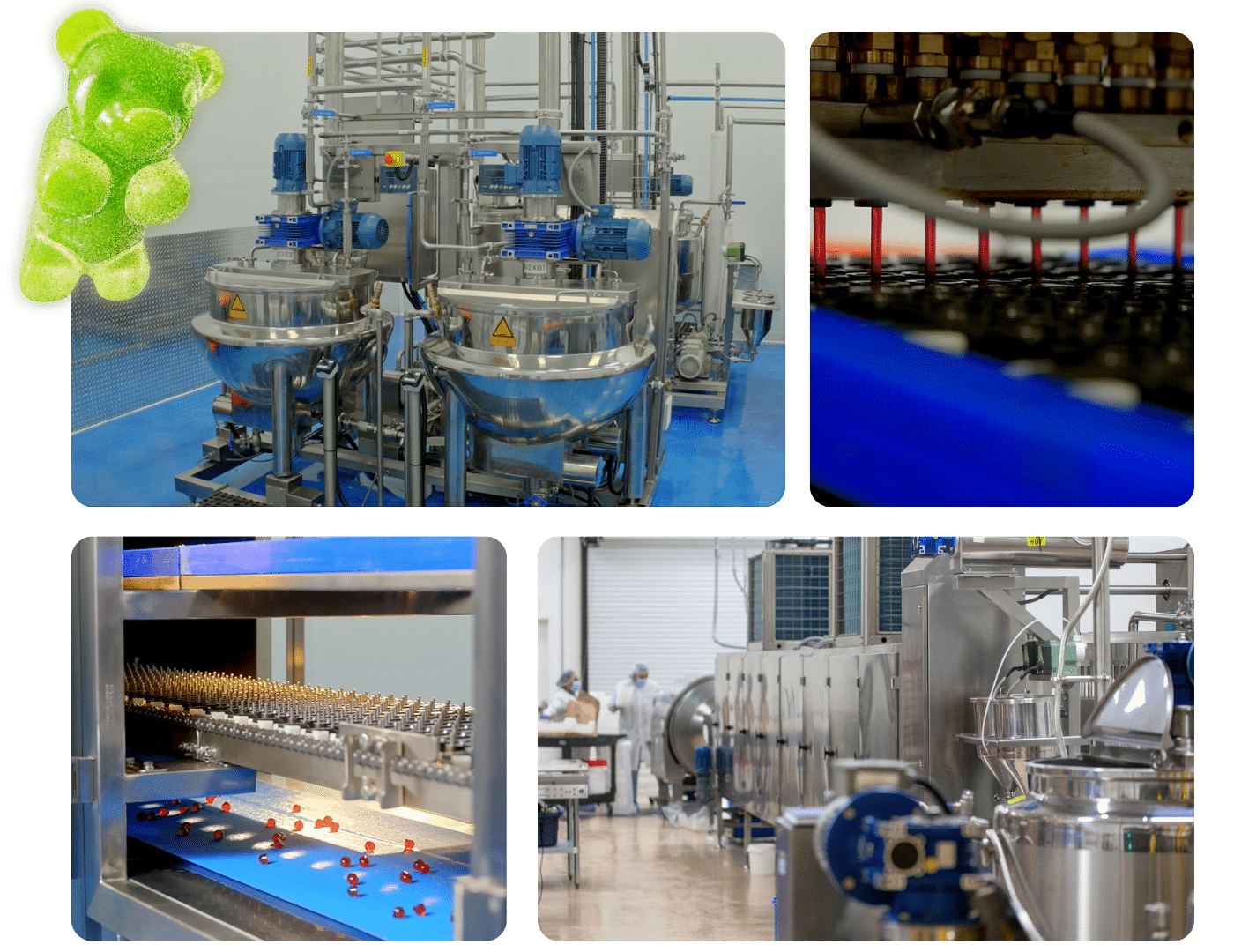
Choosing the Right Scale: Startups vs. Industry Giants
For small businesses or startups, flexible machines are key. Think of them as the versatile workhorses of your production line—easy to clean and quick to switch between recipes. These machines are perfect for test runs, letting you experiment with different flavors affordably.
On the other hand, larger enterprises benefit from high-capacity, automated lines designed for maximum efficiency. Picture a finely-tuned system producing gummies at scale with minimal downtime—an ideal setup for meeting large-scale demand like a pro! These systems often integrate with enterprise resource planning systems5 to keep everything smooth sailing.
Precision and Efficiency: No Room for Error
In the world of gummies, precision is everything. Whether it’s fortified gummies packed with vitamins or those simply meant to delight, dosing must be spot on. Machines with advanced filling and weighing capabilities ensure each gummy meets TGA’s stringent consistency standards. Think of these machines as the maestros of measurement, ensuring every batch hits the right note.
Adaptability and Upgradability: Future-Proof Your Investment
With regulations and technology advancing at lightning speed, opt for modular systems that can evolve with your business. This adaptability ensures you stay competitive as new requirements and capabilities emerge. It’s like a custom-built Lego set—always ready for the next upgrade!
Energy and Operational Efficiency: Go Green
Australia isn’t just about cuddly koalas; it’s also about keeping things eco-friendly. Machines that boast energy efficiency can significantly cut costs—saving the planet while saving your wallet! Look for options that use less water and chemicals, aligning with environmental compliance standards6 and boosting sustainability.
Expert Help: SaintyCo at Your Service
Feeling a bit overwhelmed? That’s where SaintyCo comes in—your dependable partner on this journey! From From facility layout planning7 to recipe development, we’re here to support you at every step. We even provide staff training to help your team operate machinery with confidence and skill. Think of us as a guardian angel with a toolkit of solutions!
High-capacity machines are ideal for gummy startups.False
Startups benefit from flexible machines, not high-capacity ones.
Modular systems help future-proof gummy manufacturing.True
Modular systems allow upgrades as regulations and technologies change.
Why Is Post-Market Monitoring Crucial for Compliance?
You’ve crossed the T’s and dotted the I’s, but the work isn’t over once your gummies hit the market.
Post-market monitoring is essential for maintaining compliance as it involves ongoing surveillance of product safety and effectiveness. This helps identify potential issues like adverse reactions, ensuring products continually meet regulatory standards and consumer expectations.

Continuous Monitoring: Keeping a Close Watch
After your gummy supplements hit the market, they still need careful oversight. Post-market surveillance serves as a protective measure, helping you identify any adverse reactions or quality concerns. It’s not about over-caution; it’s about ensuring confidence and safeguarding your brand's reputation.
Imagine launching a product only to discover unexpected reactions down the line. Regular monitoring catches these issues early, helping you avoid PR challenges and potential regulatory penalties.
Regulatory Alignment: Staying in TGA's Good Books
The Therapeutic Goods Administration (TGA) expects companies to uphold safety standards consistently. By actively engaging in post-market surveillance, you demonstrate commitment to compliance and regulatory alignment8. This not only keeps you in the TGA's good graces but also builds consumer trust.
Consistent updates and reports to the TGA demonstrate a proactive, rather than reactive, approach—always a stronger position with regulators. It’s like regularly servicing your car instead of waiting for a breakdown.
Consumer Confidence: Building Trust That Lasts
Consumers rely on your products being safe and effective, and ongoing post-market monitoring ensures you consistently meet—and exceed—those expectations. A well-monitored product leads to satisfied customers who keep coming back, and nothing is more powerful than word-of-mouth marketing.
Think of monitoring as an investment in your brand’s future. By avoiding potential issues and demonstrating a commitment to quality and safety, you turn customers into loyal fans.
Tools of the Trade: Tech for Tracking
In today’s digital world, technology can be your strongest ally. Advanced tracking systems and analytics software can streamline the monitoring process, with real-time data alerts catching potential issues faster than you can say “pass the gummies.”
Consider implementing automated alerts for specific quality markers or adverse reactions. The right tools turn monitoring from a manual task into a seamless, efficient part of your operations.
Post-market monitoring prevents PR nightmares.True
Monitoring helps catch issues early, avoiding public relations crises.
TGA requires no post-market surveillance for compliance.False
TGA expects ongoing surveillance to ensure safety and compliance.
Conclusion
Setting up a compliant gummies factory in Australia requires careful planning and adherence to TGA standards. Align your processes with regulations, invest in the right machinery, and seek expert guidance for a smooth operation.
With GummyGenix by SaintyCo, you’re backed by over 23 years of pharma grade machine manufacturing experience and a full suite of tailored solutions. Our advanced gummy manufacturing equipment can be customised for Australian market, supporting every stage of your production with compliance-focused machinery, expert guidance, and reliable technical support.
Ready to launch or enhance your gummy production? Contact GummyGenix by SaintyCo today to discover how our solutions can elevate your production line for lasting success in the local and global market. Let’s make your vision a reality—together.
-
Discover why listing suits lower-risk products for easier market access.: For listed medicines, the mandatory steps in manufacture are: Manufacture of dosage form (for example, tablet). Packaging & labelling. Release for supply. ↩
-
Understand GMP standards crucial for TGA registration success.: Good Manufacturing Practice (GMP) describes a set of principles and procedures that when followed helps ensure that therapeutic goods are of high quality. ↩
-
Discover how GMP-compliant machinery boosts efficiency in gummy production.: Compliance and Construction: The machine adheres to Good Manufacturing Practices (GMP), ensuring hygienic and safe production. It is built with ... ↩
-
Learn how GMP certification facilitates international market access.: GMP certification can be applied to all operators in food, ingredient or pet food supply chains – from production and packaging to retail, logistics and storage ... ↩
-
Discover how ERP systems streamline manufacturing processes effectively.: ERP implementation in manufacturing helps producers overcome challenges by providing visibility and oversight into operations. ↩
-
Learn about sustainable practices in Australian manufacturing.: We target serious non-compliance against national environment legislation. Issues we focus on include: protection of threatened plants, ... ↩
-
Explore services that optimize your manufacturing facility layout.: Design Systems, Inc. specializes in facility layout design. Our engineers and consultants can update, manage and maintain your overall shop layouts. ↩
-
Learn how to align with TGA's stringent surveillance expectations.: All adverse events of devices supplied in Australia are required to be reported to the TGA. However, an adverse event that occurs in Australia for a device that ... ↩







